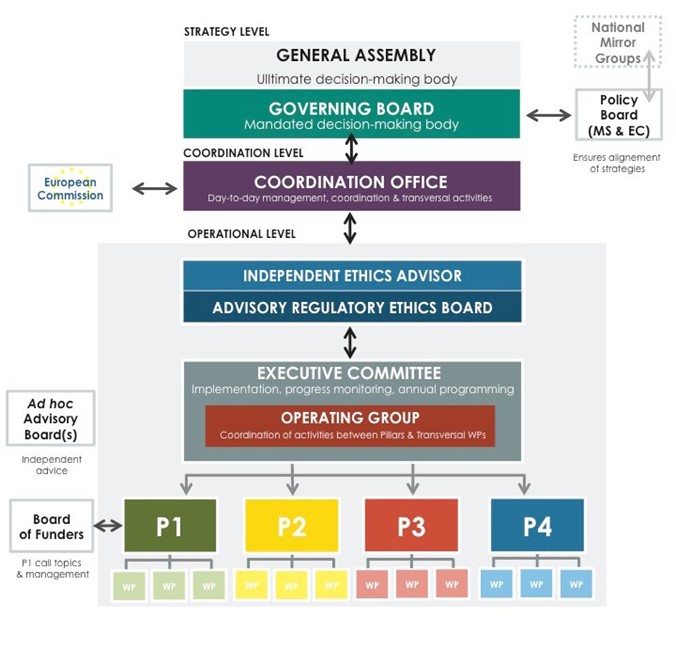
General Assembly: The General Assembly is composed of all beneficiaries of the EJP RD. The General Assembly will be the ultimate decision making body dealing with extraordinary decision requiring vote of all members like inclusion of new ones or exclusion of a nonperforming partner. For other decisions the vote will be centralized and put in hands of Governing Board. Voting procedure: The GA will strive to reach consensus and if consensus cannot be reached, the GA will vote on different options. GA Quorum and voting arrangements will be elaborated on in the Consortium Agreement.
Governing Board: The Governing Board (GB) is the highest decision making body to approve the organization, work programme, the Annual Work Plan of the following year, the structure and information flow and the political outcome of the EJP RD. The Governing Board consists of one representative per participating country, one representative of ERNs, one representative of participating EU infrastructures, one patient organization representative (EURORDIS), one representative of Orphanet and one representative of other non-profit organizations.
Coordination Office: The Coordination Office based at INSERM consists of the coordinator, two senior and two junior project managers, communication officer, financial officer and assistant. The CO is the central point and carry out the day-to-day operational management by supporting EJP RD partners.
Independent Ethics Advisor: With the aim to comply with the European ethical and legal frameworks, an independent expert is appointed as an “Ethics Advisor” in charge of advising on and monitoring ethical/legal/regulatory issues of the EJP RD, of the Data Management Plan, as well as of the projects founded within EJP RD to ensure that the ethics managementof the projects are carried out within the H2020 framework.
Advisory Regulatory Ethics Board: In order to ensure compliance of the project and its activities with ethical and regulatory requirements, a project ‘Advisory Regulatory Ethics Board’ (AREB) has been set up. It consists of internal EJP RD ethics/regulatory/legal experts, including members with recognised expertise from involved European RIs. It ensures compliance with all international and European relevant rules and ethical norms, including but not limited to fundamental and patients’ rights, data management, protection and confidentiality, of the EJP RD as well as of the projects founded within EJP RD. In particular, the AREB is in charge of overseeing ethical issues and regulatory procedures envisaged in the EJP RD four Pillars and by assisting partners.
Executive Committee: The Executive Committee (ExCom) composed of the Coordinator, project officer, Pillar leaders, patients and ERN representatives and all WP leaders) will be the forum to discuss details of the EJP RD progress, annual work plans and related resource utilization.
Operating Group: In order to coordinate the support and swift communication within and across pillars, the pillar leaders together with coordinator and project managers will form the Operating Group (OG). The OG is the driving force for the elaboration of the Annual Work Plans.
Satellite Boards
Policy Board: The members of the EJP RD are aware of the importance of continuous feedback and dialogue with relevant national, European and international stakeholders. This is necessary to translate efficiently the specific needs and strategy represented by countries or European Commission as well as the outcomes of the EJP RD. The Policy Board has a major role in ensuring this dialogue and translation through its participation in EJP RD strategy and sustainability development. The Policy Board is constituted from:
● Representatives of national ministries of research and health;
● Representatives of European Commission Directorates: DG RTD, DG Santé, DG Connect;
● Representative of the pharmaceutical industry and public-private initiatives (e.g. European Federation of Pharmaceutical Industries and Associations, EFPIA; Innovative Medicines Initiative, IMI);
● Representative of EuropaBio;
● Representative of regulatory authorities (e.g. European Medicines Agency, EMA, esp. Committee for Orphan Medicinal Products, COMP, EuNetHTA); and
● Chair and vice-chair of the International Rare Diseases Research Consortium (IRDiRC).
Meetings: Once a year
National mirror groups (NMG): Participating countries are strongly advised to constitute NMG, bringing together the national representatives of the EJP RD and other relevant RD stakeholders. The role of the NMGs is to ensure national coordination, contribute to the objectives of the EJP RD and benefit from it. The creation and composition of a NMG is at the discretion of each participating country. NMGs include representatives of the National plan for RD, national nodes of the European Reference Networks, relevant national authorities and research institutions (whether participating to the EJP RD or not), as well as the relevant national partners of the EJP RD and GB member that report NMG views and positions during GB meetings. Although not mandatory, the establishment of National Mirror Groups ensures that national activities, strategies and needs are taken into account when taking decisions at the EJP RD level and when designing the annual work plans. Please see the NMG Terms of Reference and NMG Country examples for more details.
Board of funders: In order to ensure the independence of joint transnational calls management (Pillar 1), the final decisions on call topics and implementation of calls are taken autonomously by the Board of Funders (BOF). The composition of Board of Funders is variable depending on the configuration of funding bodies participating in the joint transnational calls. BOF is chaired by the Pillar 1 leaders.
Meetings: Ad hoc meetings before and after the launch of each joint transnational call for proposals
Ad hoc Advisory Board (AB): The ad hoc Advisory Board(s) is constituted on demand of BOF or Pillar 2 and 4 leaders in relation to pilot projects foreseen. The AB consists of international experts and RD stakeholders to include their knowledge and experience and use their input for the definition of the research strategy for joint transnational calls or pilot projects.
| INSTITUTION ACRONYM | INSTITUTION | REPRESENTATIVE AND PROXY |
|---|---|---|
| ACU/ACURARE | ACIBADEM UNIVERSITESI | Osman Ugur Sezerman |
| AFM | ASS FRANCAISE CONTRE LES MYOPATHIES | Alexandre Mejat |
| AIT | AIT AUSTRIAN INSTITUTE OF TECHNOLOGY GMBH | Guenter Schreier |
| AKA | SUOMEN AKATEMIA | Heikki Vilen |
| AMC | ACADEMISCH MEDISCH CENTRUM BIJ DE UNIVERSITEIT VAN AMSTERDAM | Ronald Cornet |
| ANR | AGENCE NATIONALE DE LA RECHERCHE | Philippe Bouvet |
| AOUP | AZIENDA OSPEDALIERO UNIVERSITARIA PISANA | Rosaria Talarico |
| APHP | ASSISTANCE PUBLIQUE - HOPITAUX DE PARIS | Rima Nabbout and Alain Verloes |
| BBMRI | BIOBANKS AND BIOMOLECULAR RESOURCESRESEARCH INFRASTRUCTURE CONSORTIUM(BBMRI-ERIC) | Jens Habermann |
| BLACKSWAN | BLACKSWAN FOUNDATION | Olivier Menzel |
| CHARITE | CHARITE - UNIVERSITAETSMEDIZIN BERLIN | Fabian Prasser Thierry Meurers (proxy) |
| CIHR | CANADIAN INSTITUTES OF HEALTH RESEARCH | Christopher McMaster |
| CLB | CENTRE ANTICANCEREUX LEON BERARD | Jean-Yves Blay and Isabelle Ray-Coquard |
| CNAG-CRG | FUNDACIO CENTRE DE REGULACIO GENOMICA | Ivo Gut |
| CSO-MOH | MINISTRY OF HEALTH | Avi Israeli |
| CVBF | CONSORZIO PER VALUTAZIONI BIOLOGICHE E FARMACOLOGICHE | Bonka Georgieva |
| DFG | DEUTSCHE FORSCHUNGSGEMEINSCHAFT | |
| DLR | DEUTSCHES ZENTRUM FUER LUFT - UND RAUMFAHRT EV | Ralph Schuster |
| EATRIS | EATRIS ERIC | Anton Ussi |
| ECRIN/PedCRIN | ECRIN EUROPEAN CLINICAL RESEARCH INFRASTRUCTURE NETWORK | Marta Del Alamo |
| EKUT | EBERHARD KARLS UNIVERSITAET TUEBINGEN | Holm Graessner |
| EMBL | EUROPEAN MOLECULAR BIOLOGY LABORATORY | Juan Arenas Marquez |
| EORTC | EUROPEAN ORGANISATION FOR RESEARCH AND TREATMENT OF CANCER AISBL | Stéphane Lejeune |
| ERASMUS MC | ERASMUS UNIVERSITAIR MEDISCH CENTRUM ROTTERDAM | Irene M.J. Mathijssen |
| EURORDIS | EURORDIS - EUROPEAN ORGANISATION FOR RARE DISEASES ASSOCIATION | Roseline Favresse |
| FCT | FUNDACAO PARA A CIENCIA E A TECNOLOGIA | Rita Cavaleiro, Pedro Miguel Ferreira (Proxy) |
| FFRD | FONDATION MALADIES RARES | Daniel Scherman |
| FGB | FONDAZIONE PER LA RICERCA FARMACOLOGICA GIANNI BENZI ONLUS | Viviana Giannuzzi |
| FNR | FONDS NATIONAL DE LA RECHERCHE | Sean Sapcariu |
| FNRS | FONDS NATIONAL DE LA RECHERCHE SCIENTIFIQUE | Florence Quist, Agnès Roba (Proxy) |
| FRQS | FONDS DE RECHERCHE DU QUEBEC - SANTE | Maxime Beaudoin |
| FRRB | FONDAZIONE REGIONALE PER LA RICERCA BIOMEDICA | Paola Bello |
| FTELE | FONDAZIONE TELETHON | Stefano Benvenuti Giuditta Magnifico (proxy) |
| FWF | FONDS ZUR FÖRDERUNG DER WISSENSCHAFTLICHEN FORSCHUNG | Stephanie Resch |
| FWO | FONDS VOOR WETENSCHAPPELIJK ONDERZOEK-VLAANDEREN | Kristien Peeters |
| GSRT | GENIKI GRAMMATIA EREVNAS KAI TECHNOLOGIAS | Sofia Dimitropoulou |
| GUF | JOHANN WOLFGANG GOETHE-UNIVERSITATFRANKFURT AM MAIN | Holger Storf |
| HRB | THE HEALTH RESEARCH BOARD | Eileen Treacy |
| HUGEN | HACETTEPE UNIVERSITESI | Ece Akhan Güzelcan |
| HUS | HOPITAUX UNIVERSITAIRES DE STRASBOURG | Hélène Dolfus |
| IMAGINE | IMAGINE INSTITUT DES MALADIES GENETIQUES NECKER ENFANTS MALADES FONDATION | Stanislas Lyonnet |
| INFRAFRONTIER | INFRAFRONTIER GMBH | Michael Raess |
| INSA | INSTITUTO NACIONAL DE SAUDE DR. RICARDO JORGE | Sandra Alves |
| INSERM | INSTITUT NATIONAL DE LA SANTE ET DE LA RECHERCHE MEDICALE | Karine Groussard |
| IOR | ISTITUTO ORTOPEDICO RIZZOLI | Luca Sangiorgi |
| IPCZD | INSTYTUT POMNIK CENTRUM ZDROWIA DZIECKA | Krystyna Chrzanowska |
| ISCIII | INSTITUTO DE SALUD CARLOS III | Eva Bermejo-Sanchez |
| ISS | ISTITUTO SUPERIORE DI SANITA | Claudio Carta |
| IT | INSERM TRANSFERT SA | Catherine Clusel |
| IT MoH | MINISTERO DELLA SALUTE | Chiara Ciccarelli Anna Ceccarelli (proxy) |
| KU Leuven | KATHOLIEKE UNIVERSITEIT LEUVEN | Eric Legius |
| LBG | LUDWIG BOLTZMANN GESELLSCHAFT GMBH | Christoph Bock |
| LMT | LIETUVUOS MOKSLO TARYBA | Zivile Ruzele |
| LUMC | ACADEMISCH ZIEKENHUIS LEIDEN | Marco Roos and Alberto M. Pereira |
| MEYS | MINISTRY OF EDUCATION YOUTH AND SPORTS | Judita Klosakova |
| MoSAE | SOTSIAALMINISTEERIUM | Mari Teesalu |
| MUG | GDANSKI UNIWERSYTET MEDYCZNY | Aleksandra Zurowska |
| MUW | WARSZAWSKI UNIWERSYTET MEDYCZNY | Piotr Fiedor |
| NCBR | NARODOWE CENTRUM BADAN I ROZWOJU | Marcin Chmielewski |
| NKFIH | NEMZETI KUTATASI FEJLESZTESI ES INNOVACIOS HIVATAL | Elod Nemerkényi |
| SAS | SLOVENSKA AKADEMIA VIED | Silvia Kecerova |
| SERMAS | SERVICIO MADRILENO DE SALUD | Eduardo Lopez Granados |
| SNSF | SCHWEIZERISCHER NATIONALFONDS ZUR FORDERUNG DER WISSENSCHAFTLICHEN FORSCHUNG | Tobias Braun |
| SRC | VETENSKAPSRADET - SWEDISH RESEARCH COUNCIL | Louise Rügheimer |
| TEDDY | "TEDDY - EUROPEAN NETWORK OF EXCELLENCE FOR PAEDIATRIC CLINICAL RESEARCH" | |
| TUBITAK | TURKIYE BILIMSEL VE TEKNOLOJIK ARASTIRMA KURUMU | Jale Sahin |
| TuscReg | REGIONE TOSCANA | Katia Belvedere |
| UKA | UNIVERSITAETSKLINIKUM AACHEN | Ralf-Dieter Hilgers |
| UKL-HD | UNIVERSITAETSKLINIKUM HEIDELBERG | Franz Schaefer |
| UKLFR | UNIVERSITAETSKLINIKUM FREIBURG | Hanns Lochmüller |
| ULEIC | UNIVERSITY OF LEICESTER | Anthony J. Brookes |
| ULIV | THE UNIVERSITY OF LIVERPOOL | Mark Turner |
| UM | UNIVERSITEIT MAASTRICHT | Chris Evelo |
| UMCG | ACADEMISCH ZIEKENHUIS GRONINGEN | Morris A. Swertz |
| UNEW | UNIVERSITY OF NEWCASTLE UPON TYNE | Volker Straub |
| UNIUD | UNIVERSITÀ DEGLI STUDI DI UDINE | Maurizio Scarpa |
| UPM | UNIVERSIDAD POLITECNICA DE MADRID | Mark Wilkinson |
| VINNOVA | VERKET FÖR INNOVATIONSSYSTEM | Gunnar Sandberg |
| VUHSK | VIESOJI ISTAIGA VILNIAUS UNIVERSITETO LIGONINE SANTAROS KLINIKOS | Birute Tumiene |
| ZonMw | ZORGONDERZOEK NEDERLAND ZON | Harald Moonen |
EUROPEAN ORGANISATIONS
EUCOPE: Diego Ardigò
EuropaBio: Adrien Samson
IRDiRC: David Pearce and Samantha Parker
EURORDIS: Valentina Bottarelli
EMA: tbc
DG Santé: tbc
DG RTD: Carmen Laplaza Santos
DG CNECT: Szymon Bielecki
ARMENIA
Ministry of Education: Naira Ayvazan
AUSTRIA
Ministry of Health and Women’s affairs: Lovro MARKOVIĆ
BULGARIA
Ministry of Health: Irina Kovacheva
CANADA
Health Products and Food Branch in Health Canada: Alysha Croker
Ministry of Innovation, Science and Economic Development: Christopher McMaster, Jeanne Egar
CROATIA
Ministry of Health and Social Welfare: Ingeborg Barisic
CZECH REPUBLIC
Ministry of Education, Youth and Sports: Judita Klosakova
Ministry of Health: Pavla Dolezalova
ESTONIA
Ministry of Social Affairs: Heli Paluste
FRANCE
Ministry of Solidarity and Health: Jérôme Weinbach
Ministry of Education, Research, and Innovation: Bertrand Schwartz
GEORGIA
Ministry for Health: Ekaterine Khmaladze
GERMANY
Ministry of Health: Cornelia Blaschke
Ministry of Education and Research: Tonia Bieber
GREECE
Ministry of Education, Research and Religious Affairs: Sossana Kolyva
HUNGARY
Ministry of Health: Gyorgy Pfliegler
ISRAEL
Ministry of Health: Avi Israeli
ITALY
Ministry of Health: Gaetano Guglielmi
Ministry of Education, University, and Research: Alessandra Renieri
LATVIA
Ministry of Education and Science: Janis Ancans
LITHUANIA
Ministry of Education, Science and Sport: Daine Denisoviene
Ministry of Health: Anželika Balčiūnienė
MALTA
Ministry for Health: Miriam Azzopardi
NETHERLANDS
Ministry of Health, Welfare and Sport: Maaike Wijnhoud
NORWAY
Ministry of Health and Care Services: Nora Gamst
POLAND
Ministry of Health: Jakub Adamski
Ministry of Science and Higher Education: Ryszard Rzepecki
PORTUGAL
Ministry of Health: Válter Fonseca
Ministry of Science, Technology and Higher Education: Patrícia Maciel
SLOVAKIA
Ministry of Health: Tatiana Foltánová
Ministry of Education, Science, Research and Sport: Richard Imrich
SPAIN
Ministry of Health, Social Services and Equality: Pilar Aparicio Azcárraga
SWITZERLAND
State Secretariat for Education, Research and Innovation (SERI): Myriam Cevallos
TÜRKIYE
Ministry of Health: Onur Burak Dursun
UNITED KINGDOM
Department of Health: Monika Preuss
| PARTNER ACRONYM | COUNTRY | ROLE | NAME |
|---|---|---|---|
| INSERM | France | Coordinator | Daria Julkowska |
| INSERM | France | IT GGB director | Emmanuelle Genin |
| INSERM | France | Pillar 2 coleader | Ana Rath |
| DLR | Germany | Pillar 1 coleader | Ralph Schuster |
| VUHSK | Lithuania | Pillar 3 coleader | Biruté Tumiene |
| ZON (ZonMw) | Netherlands | Pillar 1 coleader | Sonja van Weely |
| APHP | France | Pillar 4 coleader | Rima Nabbout |
| UKL-HD | Germany | Pillar 2 coleader | Franz Schaefer |
| EATRIS | Infrastructure | Pillar 4 coleader | Anton Ussi |
| EURORDIS | EU organisation (France) | Pillar 3 coleader | Roseline Favresse |
| INSERM | France | WP1 | Daria Julkowska |
| ISCIII | Spain | WP2 | Eva Bermejo-Sanchez |
| ISS | Italy | WP2 | Claudio Carta |
| EATRIS | Infrastructure | WP3 | Anton Ussi |
| FGB | Italy | WP4 | Viviana Giannuzzi |
| FTELE | Italy | WP4 | Barbara Sanavio |
| INSERM | France | WP5 | Tanguy Onakoy |
| DLR | Germany | WP6 | Ralph Schuster |
| ANR | France | WP6 | Florence Guillot |
| ZON (ZonMw) | Netherlands | WP7 | Sonja van Weely |
| FFRD | France | WP8 | Christine Fetro |
| CSO/MoH | Israel | WP9 | Irit Allon |
| INSERM | France | WP10 | Ana Rath |
| ULEIC | United Kingdom | WP10 | Anthony Brookes |
| INSERM | France | WP11 | Ana Rath |
| CNAG-CRG | Spain | WP11 | Sergi Beltran |
| ULEIC | Spain | WP12 | Anthony Brookes |
| LUMC | Netherlands | WP12 | Marco Roos |
| UKL-HD | Germany | WP13 | Franz Schaefer |
| UM | Netherlands | WP13 | Chris Evelo |
| ISS | Italy | WP14 | Claudio Carta |
| EURORDIS | EU organisation (France) | WP15 | Roseline Favresse |
| FFRD | France | WP16 | Magda Granata |
| EKUT (ERN-RND) | Germany | WP17 | Holm Graessner |
| VUHSK | Lithuania | WP18 | Biruté Tumiene |
| IPCZD (CMHI) | Poland | WP18 | Krystyna Chrzanowska |
| EURORDIS | EU organisation (France) | WP18 | Roseline Favresse |
| EATRIS | Infrastructure | WP19 | Anton Ussi |
| FTELE | Italy | WP19 | Barbara Sanavio |
| APHP | France | WP20 | Rima Nabbout |
| UKA | Germany | WP20 | Ralf-Dieter Hilgers |
| HSK | Germany | WP20 | Maurizio Scarpa |
Members
Marta del Alamo, European Clinical Research Infrastructure Network (ECRIN), France
Viviana Giannuzzi, Fondazione per la ricerca farmacologica Gianni Benzi Onlus (FGB), Italy
Michaela Th. Mayrhofer, Biobanks And Biomolecular Resources research Infrastructure Consortium (BBMRI- ERIC), Austria
Rob Hooft, European life-sciences Infrastructure for biological Information (EMBL-EBI –DTL PROJECTS– ELIXIR/ELIXIR-NL) The Netherland
Giovanni Migliaccio, Consorzio per Valutazioni Biologiche e Farmacologiche (CVBF), Italy
Sonia Gueguen, Institut National De La Sante Et De La Recherche Medicale for Rare Disease Cohorts (RaDiCo-Inserm), France
Irene Schlünder, Biobanks And Biomolecular Resources research Infrastructure Consortium (BBMRI- ERIC), Austria
Deputy members
Annalisa Landi, Fondazione per la ricerca farmacologica Gianni Benzi Onlus (FGB), Italy
Petr Holub, Biobanks And Biomolecular Resources research Infrastructure Consortium (BBMRI- ERIC), Austria
Independent ethics advisors
Anne Demoisy
Joana Namorado